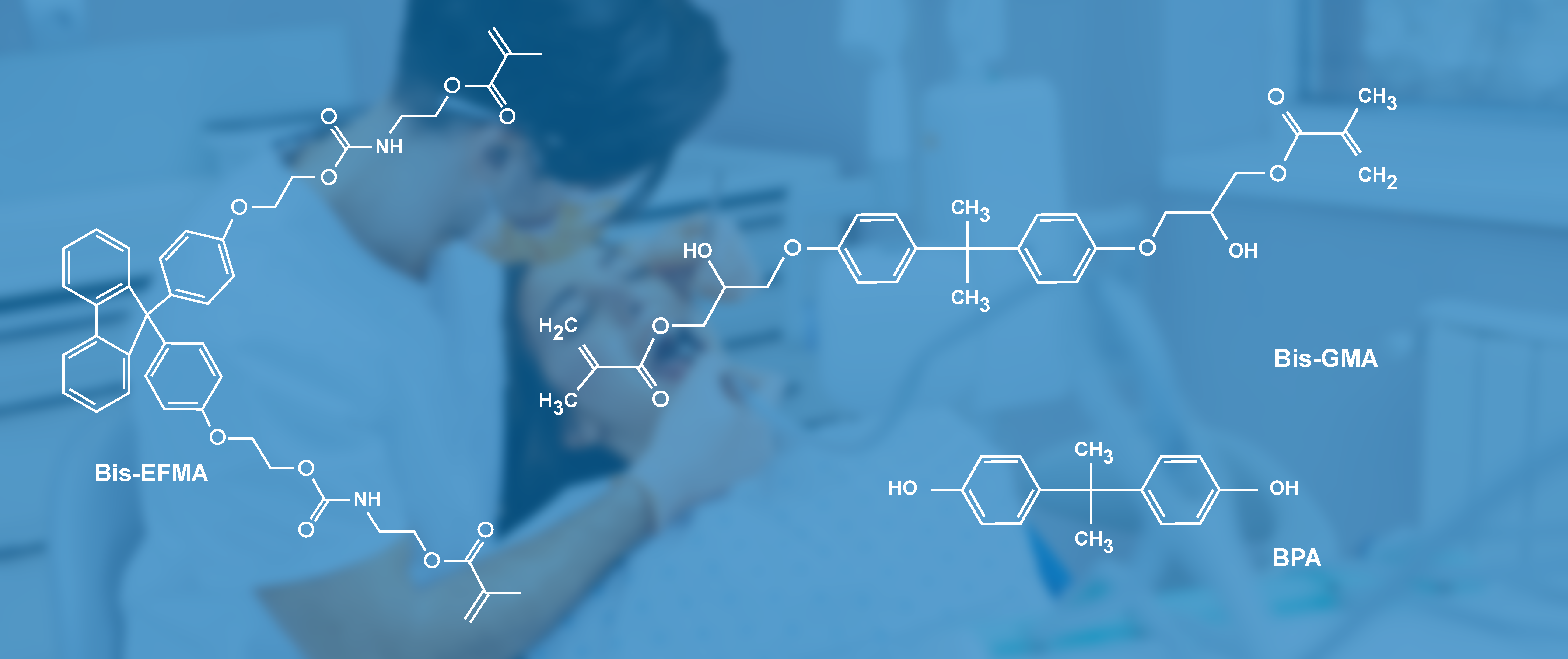
New BPA-free monomer for dental materials

A new BPA-free monomer may substitute Bis-GMA as major monomer in commercial dental composites.
The degree of conversion and the material properties of an experimental composite were of similar values as for a commercial material.
NIOM guest-researcher Jingwei He has successfully synthesized a BPA-free monomer, which has been evaluated for experimental materials. The conclusion is that the new monomer has potential to be used in commercial dental composites.
Bisphenol A (BPA) has been reported as one of many endocrine-disrupting compounds and can be found as a contamination in some dental materials, in particular, fissure sealants and composites albeit in small amounts. The chemical structure of BPA has similarities to that of estrogens and may therefore induce an unwanted biological response. The source of BPA in dental materials is usually the common monomer Bis-GMA. Investigations to find monomers that can replace Bis-GMA in these materials are therefore of interest, to rule out dental materials as a potential source of BPA-exposure.
In a recent project at NIOM, Dr. He synthesized a new BPA-free monomer abbreviated Bis-EFMA. This monomer does not have the core structure similar to BPA that Bis-GMA does (see figure). Dr. He then evaluated critical properties of composite materials
based on the new monomer in comparison to a similar material with Bis-GMA and to a commercial composite.
The degree of conversion of the experimental composite with Bis-EFMA was the same as for the commercial material and for the Bis-GMA-based composite. This indicates that the material properties also will show similar values to the commercial materials. Indeed, all the measured properties (except flexural strength after water immersion) were similar to or better than those of the commercial material. In particular, a lower polymerization shrinkage (2.1 vol-% vs 2.6 vol-%) and a higher depth of cure (5.4 mm vs. 3.4 mm) were found for the Bis-EFMA-composite.
An initial screening of cytotoxicity using the MTT-test showed greater viability of cells exposed to extract from the Bis-EFMA-based composite (viability ca. 87 %) compared to the commercial material (viability ca. 61 %) and the Bis-GMA-based composite
(viability ca. 41 %).
REFERENCE:
Jingwei He, Hilde M. Kopperud.
Preparation and characterization of Bis-GMA-free dental composites with dimethacrylate monomer derived from 9,9-Bis[4-(2-hydroxyethoxy)phenyl]fluorine.
Dental materials 2018; 34: 1003-13.