Faulty bond strength measurements?
No change in core temperature.
60 second dwell is often required.

The most commonly used bonding stress method for testing adhesives and cement, the thermal cycling, jeopardizes the results if not done with caution. This leads to a more favorable bond strength than the true value.
The thermal cycling method aims to stress the adhesive area by changing the temperature in the test specimens from 5 °C to 55 °C. These temperatures are supposed to be reached by cyclic placing of the test specimens in water baths of 5 °C and 55 °C for a certain dwell time.
Studies showed that by using the most commonly used dwell time of 20 sec, test specimens of teeth or composite filling material (10x10x10 mm) never reached the water bath’s temperature in the core of the specimen.
Micro tensile testing after thermal cycling revealed that samples taken from the core of such specimens had significant higher bond strength than samples taken from the outer part. In short, outer samples were more stressed by the temperature change than those of the core were.
A recent study investigated the dwell time necessary for getting a homogeneous temperature change in differently sized test specimens of composite filling material and in test specimens containing tooth structure.
For most test specimens 60 sec dwell time was needed to obtain homogeneous temperature in the specimens similar to water bath temperature whereas for specimens made from tooth substance an even longer dwell time was required.
Clinical implication:
Bond strength reported after thermal cycling may not reflect the true situation and must be taken into account when reading scientific reports and promotional material.
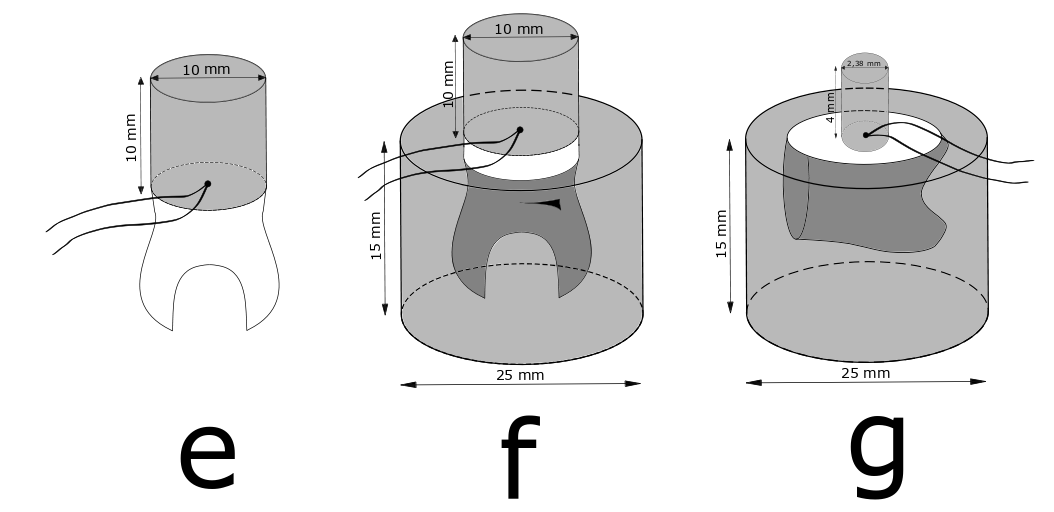
Test specimen assemblies named e, f, and g, with thermocouples placed in the centre radius between tooth and composite. Credit: Dimitri Alkarra.
______________________________________________________________________________________
Reference: SCHEER (Scientific Committee on Health, Environmental and Emerging Risks), Guidelines on the benefit-risk assessment of the presence of phthalates in certain medical devices covering phthalates which are carcinogenic, mutagenic, toxic to reproduction (CMR) or have endocrine-disrupting (ED) properties, final version adopted at SCHEER plenary on 18 June 2019.
Summary given in: Regulatory Toxicology and Pharmacology 2020; 111: 104546.
NIOM Newsletter February 2020
